Medical devices approved under the old MDD must be reassessed by a Notified Body for MDR compliance before the 26th May 2024. But you can still apply for an extension of the transition period if you meet certain conditions. What are they?
EU MDR: New reapproval deadlines issued for 2024
In 2023, the EU Commission released details of new extensions of deadlines for the reapproval of legacy devices to meet the requirements of the MDR.
And, yes. I know we’ve been here before. The EU have imposed numerous deadlines for companies that have been moved numerous times.
But this time they REALLY mean it.
Those whose legacy devices do not comply with the MDR by the 26th May 2024, or do not have a valid extension by that date will not be able to sell those devices in the EU.
So, if you haven’t yet secured re-approval from a notified body - can you apply for an extension?
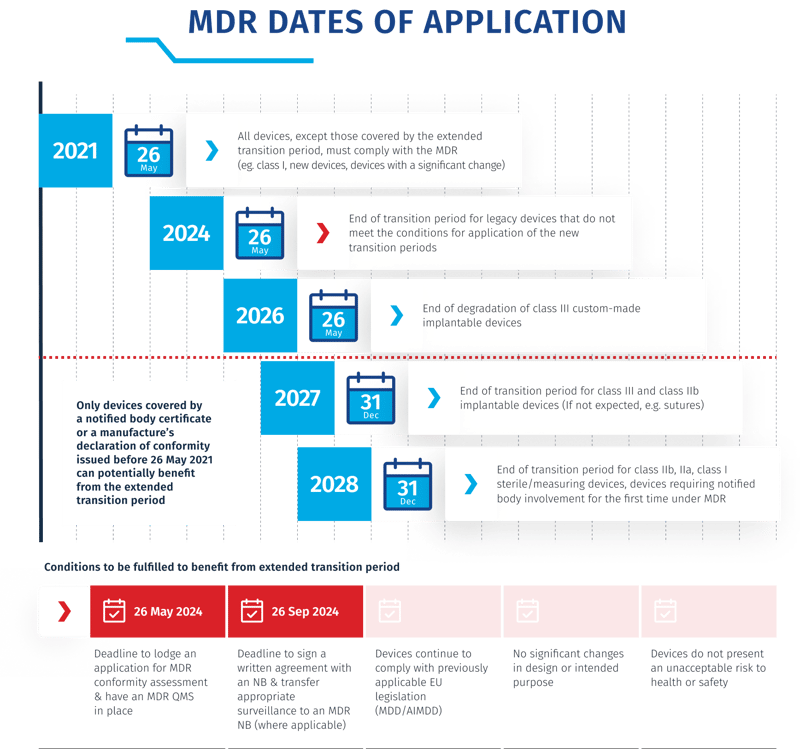
What are the new deadlines announced for transition extensions?
- Class III Custom-Made Implantable Devices: Class III custom-made implantable devices can benefit from an extended transition period until May 26, 2026.
- Class IIb Implantable Devices and Other Classes: For Class IIb implantable devices and other classes of devices, the transition period is extended until December 31, 2027.
- Other Devices: The extension also covers Class IIa, IIb devices not included in the previous point, Class I sterile devices or those with measuring functions, as well as devices classified as Class I under the directives but requiring a Notified Body assessment for the first time under MDR. These devices have an extended transition period until December 31, 2028.
But, be warned, to be granted an extension may still require considerable work on your part - so businesses who have not acted yet must do so.
What are the conditions for extension?
If devices have had no significant changes in design or intended purpose since their original approval, you can apply for an extended transition period, under the following conditions:
- Manufacturers or authorized representatives must lodge a formal application for conformity assessment in accordance with the Medical Device Regulation (MDR) no later than 26 May 2024.
- The device had a valid certificate issued under MDD or AIMDD (Active Implantable Medical Devices Directive) before May 26, 2021.
- A written agreement between the manufacturer and the notified body must be signed no later than 26 September 2024 to benefit from the extended transitional period.
- The application should clearly identify the manufacturer, the devices covered by the application, their classification, and the chosen conformity assessment procedure.
- By 26 May 2024, manufacturers must have put in place a quality management system (QMS) for every device in accordance with Article 10(9) of the MDR.
Luckily, to make matters clearer, the EU has released a new infographic to illustrate the new deadlines.
Conclusion
This time there is likely to be no reprieve.
If you have legacy devices that are still on the market but have not been reapproved by a Notified Body then you must act well before 26th May 2024 to ensure your products can still be sold in the EU.

